Abstract
Aplastic Anemia (AA) arises due to specific failure of bone marrow precursor cells or stem cells to produce adequate amount of mature hematopoietic cells. Erythropoiesis utilizes majority of iron in humans. Erythropoietic activity is fine-tuned with iron regulation since they are both intrinsically regulated. Hepcidin, the main iron regulator is the target of erythropoietic regulators like GDF15, TWSG1 and erythroferrone. Even though the dysregulation of iron balance is well understood in beta thalassemia where ineffective erythropoiesis is the main cause, regulation of iron is poorly elucidated in bone marrow failure where erythropoiesis is minimal. In this study, we analysed several iron regulatory molecules in a cohort of patients with AA and tried to understand iron regulation in bone marrow failure.
The current study investigated 77 patients with newly or recently diagnosed AA and had received less than ten transfusions from the year 2016 to 2018. Their haematological parameters were documented. Serum hepcidin was assessed using ELISA kit (DRG Diagnostics) and serum ferritin and soluble transferrin receptor (sTfR) was measured by chemiluminescent immunoassay. DNA was extracted from peripheral blood and telomere length was measured using quantitative real-time PCR (qPCR) using relative quantitation method. The association between different iron regulators were analysed using SPSS Software.
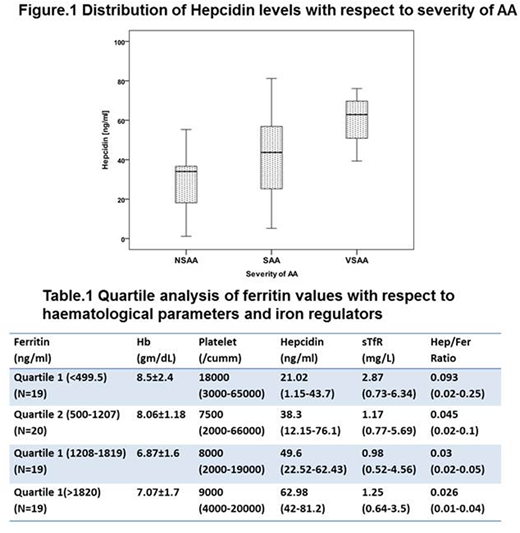
The median age of AA patients was 38 (5-72) years and the number of males and females were 49 (64%) and 28 (36%) respectively. Patients were grouped based on severity into non severe AA (NSAA) (n=17, 22%), Severe AA (SAA) (n=51, 66%) and Very Severe AA (VSAA) (n=9, 12%). Out of 77 AA patients, 50 had not received any red cell transfusion in the last one month prior to the sample collection; twenty seven had received transfusions ranging from 1 to 9. The mean haemoglobin was 7.6±1.87g/dL, with a median WBC count of 2900 (100-8400)/cu.mm and a median platelet count of 9000 (2000-66000)/ul. Serum ferritin levels ranged from 12.8 to 5728ng/ml (Median- 1208ng/ml). The median soluble transferrin receptor (sTfR) levels were 1.29mg/L (0.52-6.34). Fifty four patients (54/77, 70%) had sTfR values less than the lower limit of normal (Normal range 1.8-4.6 mg/L). Median serum hepcidin was 40.66ng/ml (1.15-81.2). Serum ferritin levels positively correlated with serum hepcidin (r=0.61, p=0.000) whereas it correlated negatively with sTfR (r=-0.408, p=0.000). No significant association was observed between the number of transfusions and ferritin levels (r=0.087, p=0.453). There was no significant difference in the ferritin levels when patients were grouped according to severity (Median Ferritin, Range - 1208, 12.8-2984ng/ml in NSAA; 1099, 61.8-5728ng/ml in SAA; 1288, 268-3824; p= 0.83). Significantly higher hepcidin levels were observed in VSAA as compared to NSAA (Figure.1, p= 0.014). There was no significant association observed between response and biochemical parameters. A quartile analysis based on ferritin values was carried out to study the effect of iron overload on haematological parameters and iron regulators (Table.1). With increasing ferritin values, hepcidin levels significantly increased (p=0.001) whereas sTfR levels were reduced (p=0.002).
In AA, decrease in erythropoietic activity as evidenced by reduced sTfR levels leads to minimal iron utilization and accumulation of iron in the tissues as ferritin. This stimulates the production of hepcidin and further exacerbates iron loading and leads to dysregulation of iron homeostasis. Increased iron in the marrow can lead to oxidative stress and may further be detrimental to the limited intrinsic haematopoiesis. Hence better understanding of iron regulation in AA may advance to effective therapeutic options.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal